

The College of Oxford partnered with the British-Swedish firm AstraZeneca to develop and take a look at a coronavirus vaccine generally known as ChAdOx1 nCoV-19 or AZD1222. A medical trial revealed the vaccine was as much as 90 % efficient, relying on the preliminary dosage. However uncertainty over the outcomes has clouded its prospects.
A Piece of the Coronavirus
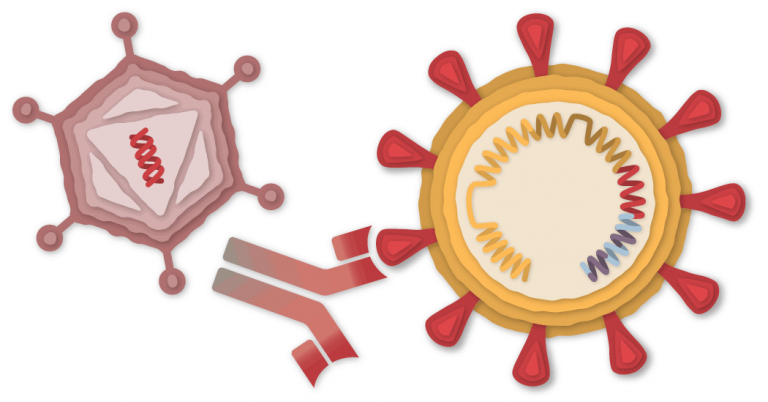
The SARS-CoV-2 virus is studded with proteins that it makes use of to enter human cells. These so-called spike proteins make a tempting goal for potential vaccines and coverings.


The Oxford-AstraZeneca vaccine relies on the virus’s genetic directions for constructing the spike protein. However not like the Pfizer-BioNTech and Moderna vaccines, which retailer the directions in single-stranded RNA, the Oxford vaccine makes use of double-stranded DNA.
DNA Inside an Adenovirus
The researchers added the gene for the coronavirus spike protein to a different virus known as an adenovirus. Adenoviruses are widespread viruses that usually trigger colds or flu-like signs. The Oxford-AstraZeneca group used a modified model of a chimpanzee adenovirus, generally known as ChAdOx1. It may possibly enter cells, however it could possibly’t replicate inside them.


AZD1222 comes out of many years of analysis on adenovirus-based vaccines. In July, the first one was permitted for normal use — a vaccine for Ebola, made by Johnson & Johnson. Superior medical trials are underway for different illnesses, together with H.I.V. and Zika.
The Oxford-AstraZeneca vaccine for Covid-19 is extra rugged than the mRNA vaccines from Pfizer and Moderna. DNA isn’t as fragile as RNA, and the adenovirus’s robust protein coat helps shield the genetic materials inside. In consequence, the Oxford vaccine doesn’t have to remain frozen. The vaccine is anticipated to final for a minimum of six months when refrigerated at 38–46 levels Fahrenheit (2–Eight levels Celsius).
Coming into a Cell
After the vaccine is injected into an individual’s arm, the adenoviruses stumble upon cells and latch onto proteins on their floor. The cell engulfs the virus in a bubble and pulls it inside. As soon as inside, the adenovirus escapes from the bubble and travels to the nucleus, the chamber the place the cell’s DNA is saved.

Virus engulfed
in a bubble

Virus engulfed
in a bubble

Virus engulfed
in a bubble





The adenovirus pushes its DNA into the nucleus. The adenovirus is engineered so it could possibly’t make copies of itself, however the gene for the coronavirus spike protein could be learn by the cell and copied right into a molecule known as messenger RNA, or mRNA.
Constructing Spike Proteins
The mRNA leaves the nucleus, and the cell’s molecules learn its sequence and start assembling spike proteins.

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments

Three spike
proteins mix
Spikes
and protein
fragments
Displaying
spike protein
fragments
A few of the spike proteins produced by the cell type spikes that migrate to its floor and stick out their suggestions. The vaccinated cells additionally break up a few of the proteins into fragments, which they current on their floor. These protruding spikes and spike protein fragments can then be acknowledged by the immune system.
The adenovirus additionally provokes the immune system by switching on the cell’s alarm methods. The cell sends out warning alerts to activate immune cells close by. By elevating this alarm, the Oxford-AstraZeneca vaccine causes the immune system to react extra strongly to the spike proteins.
Recognizing the Intruder
When a vaccinated cell dies, the particles accommodates spike proteins and protein fragments that may then be taken up by a sort of immune cell known as an antigen-presenting cell.

Presenting a
spike protein
fragment

Presenting a
spike protein
fragment

Presenting a
spike protein
fragment
The cell presents fragments of the spike protein on its floor. When different cells known as helper T-cells detect these fragments, the helper T-cells can increase the alarm and assist marshal different immune cells to struggle the an infection.
Making Antibodies
Different immune cells, known as B-cells, could stumble upon the coronavirus spikes and protein fragments on the floor of vaccinated cells. A number of of the B-cells could possibly lock onto the spike proteins. If these B-cells are then activated by helper T-cells, they are going to begin to proliferate and pour out antibodies that focus on the spike protein.

Matching
floor proteins

Matching
floor proteins

Matching
floor proteins

Matching
floor proteins

Matching
floor proteins

Matching
floor proteins

Matching
floor
proteins

Matching
floor
proteins

Matching
floor
proteins

Matching
floor proteins

Matching
floor proteins

Matching
floor proteins
Stopping the Virus
The antibodies can latch onto coronavirus spikes, mark the virus for destruction and stop an infection by blocking the spikes from attaching to different cells.
Killing Contaminated Cells
The antigen-presenting cells may also activate one other sort of immune cell known as a killer T-cell to hunt out and destroy any coronavirus-infected cells that show the spike protein fragments on their surfaces.

Presenting a
spike protein
fragment
Starting
to kill the
contaminated cell

Presenting a
spike protein
fragment
Starting
to kill the
contaminated cell

Presenting a
spike protein
fragment
Starting
to kill the
contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell

Presenting a
spike protein
fragment
Starting to kill
the contaminated cell
Remembering the Virus
The Oxford-AstraZeneca vaccine requires two doses, given 4 weeks aside, to prime the immune system to struggle off the coronavirus. Throughout the medical trial of the vaccine, the researchers unwittingly gave some volunteers solely half a dose.
Surprisingly, the vaccine mixture wherein the first dose was solely half energy was 90 % efficient at stopping Covid-19 in the medical trial. In distinction, the mixture of two full-dose photographs led to only 62 % efficacy. The researchers speculate that the decrease first dose did a greater job of mimicking the expertise of an an infection, selling a stronger immune response when the second dose was administered.

Second dose
28 days later

Second dose
28 days later

Second dose
28 days later
As a result of the vaccine is so new, researchers don’t know the way lengthy its safety may final. It’s potential that in the months after vaccination, the variety of antibodies and killer T-cells will drop. However the immune system additionally accommodates particular cells known as reminiscence B-cells and reminiscence T-cells which may retain details about the coronavirus for years and even many years.
For extra about the vaccine, see AstraZeneca’s Covid Vaccine: What You Must Know.
Vaccine Timeline
January, 2020 Researchers at the College of Oxford’s Jenner Institute start work on a coronavirus vaccine.
March 27 Oxford researchers start screening volunteers for a human trial.
April 23 Oxford begins a Section 1/2 trial in Britain.
![]()
A vial of the Oxford-AstraZeneca vaccine.John Cairns/College of Oxford/Agence France-Presse
April 30 Oxford companions with AstraZeneca to develop, manufacture and distribute the vaccine.
Might 21 The U.S. authorities pledges as much as $1.2 billion to assist fund AstraZeneca’s growth and manufacturing of the vaccine.
Might 28 A Section 2/three trial of the vaccine begins in Britain. A few of the volunteers unintentionally obtain half of the supposed dose.
June 23 A Section three trial begins in Brazil.
June 28 A Section 1/2 examine begins in South Africa.
July 30 A paper in Nature reveals the vaccine seems protected in animals and appears to stop pneumonia.
Aug. 18 A Section three trial of the vaccine begins in the United States, with 40,000 individuals.
Sept. 6 Human trials are placed on maintain round the world after a suspected opposed response in a British volunteer. Neither AstraZeneca nor Oxford announce the pause.
Sept. 8 The information about paused trials turns into public.
Sept. 12 The medical trial resumes in the U.Okay. however stays paused in the United States.
![]()
A syringe of the vaccine at a trial website in Britain.Andrew Testa for the New York Instances
Oct. 23 After investigation, the Meals and Drug Administration permits the Section three medical trial to proceed in the United States.
Nov. 23 AstraZeneca publicizes medical trial information that reveals an preliminary half dose of the vaccine seems simpler than a full dose. However irregularities and omissions immediate many questions on the outcomes.
![]()
Prime Minister Boris Johnson of Britain holds a vial of the vaccine.Pool photograph by Paul Ellis
Dec. 7 The Serum Institute of India publicizes that it has utilized to the Indian authorities for emergency use authorization of the vaccine, generally known as Covishield in India.
Dec. 8 Oxford and AstraZeneca publish the first scientific paper on a Section three medical trial of a coronavirus vaccine.
Dec. 11 AstraZeneca publicizes that it’ll collaborate with the Russian creators of the Sputnik V vaccine, which can also be made out of adenoviruses.
2021 The corporate expects to provide as much as two billion doses subsequent yr. Every vaccinated particular person would require two doses, at an anticipated worth of $three to $four per dose.
Sources: Nationwide Heart for Biotechnology Data; Nature; Lynda Coughlan, College of Maryland Faculty of Drugs.
Monitoring the Coronavirus